 |
 |

Appendix I
Absorption
by Water and Henry's Law
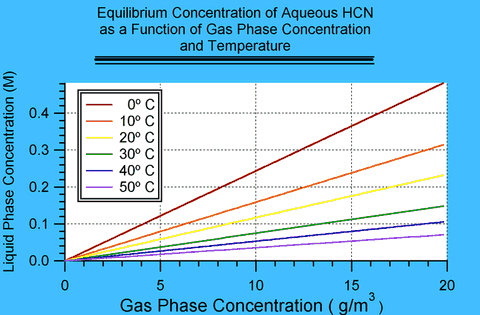
Page 32 of DuPont's Hydrogen Cyanide: Properties, Uses, Storage and Handling 1 contains a plot of the partial pressure of HCN above aqueous solutions of HCN at various concentrations and temperature. These values are equilibrium values. That means that at these concentrations the rate of HCN in the gas phase becoming absorbed into the solution is exactly balanced by the rate of HCN leaving solution into the gas phase.
Because the plot shows equilibrium values, it contains implicitly the value of partition coefficients, i.e., it is possible to obtain the equilibrium concentration of HCN in solution in water exposed to HCN in the gas phase as a given concentration and temperature. This appendix extracts those values. In DuPont's plot liquid phase concentration is expressed in weight percent and gas phase concentration in millimeters of mercury (also known as Torr); this appendix derives relationships in terms of molarity (M) and grams per cubic meter (g/m3).
These values are equilibrium values, which means that they are an upper limit to the concentration that may be found in water exposed to HCN. How fast such equilibrium establishes itself is a question of kinetics and is a much more difficult problem.
By reading the values for a given temperature from the plot, one can construct a plot of the weight percent HCN in water as a function of gas phase concentration in Torr. The relationship is linear in the region of interest; so intermediate values can be found by fitting the points with a least-squares linear regression. At 0 Torr, the concentration in water should be 0%; therefore the fits have only one free parameter, the slope. This linear relationship is known as Henry's Law and the slope can be identified with the Henry's Law constant.
Figure I.1
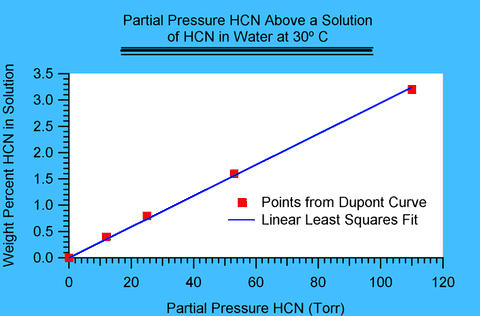
Figure I.1 shows such a plot for a temperature of 30 degrees Celsius (° C). The points have been read by eye from DuPont's plot. Notice that the fit to a line is excellent and is likely to average out slight deviations in the estimates of values in DuPont's plot. The slope in this case was found to be 0.029 percent/Torr.